R&D
Realheart’s research and development work aims to bring Realheart®️ TAH to the market in accordance with requirements set by the US Food and Drug Administration (FDA).
The idea behind Realheart®️ TAH is based on fluid dynamics studies performed at the Swedish Royal Institute of Technology (KTH) during 2002-2005 with the aim of constructing an artificial heart that mimics the blood flow of the human heart. By imitating the human heart’s primary pumping principle, a physiological pressure and flow is created that should reduce the risk of blood clots and provide energy-efficient pumping. These factors are important to give the patient a good quality of life.
Over the years, Realheart has developed a series of prototypes and gradually solved one challenge after another. Blood circulation, pump function, pressure, and pulse generation have been verified in ethically approved animal experiments.
Development Plan
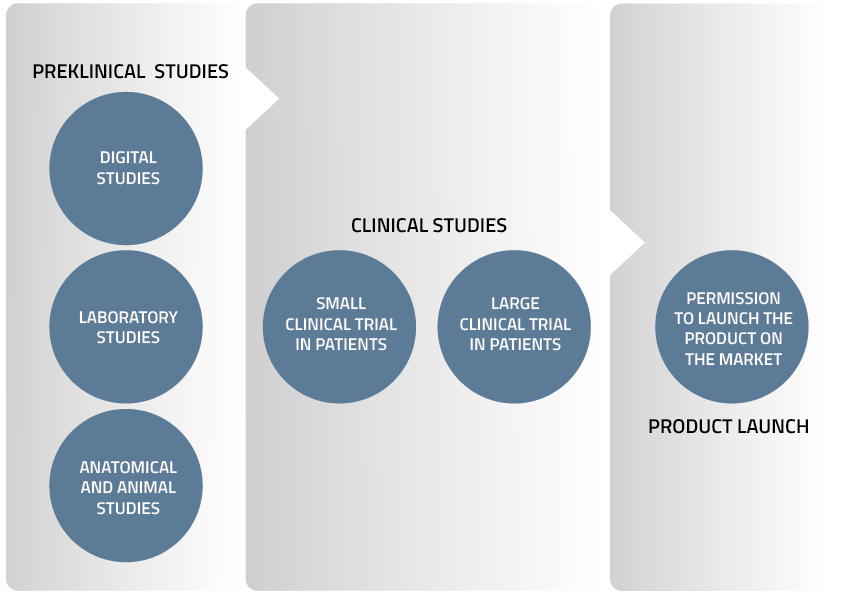
Preclinical Studies
Digital Studies
- Virtual implants in animals and human heart failure patients in collaboration with Virtonomy GmbH
- Computer simulation of blood flow in the pump (Computational Fluid Dynamics, CFD) in collaboration with University of Bath under the direction of Dr. Katharine Fraser
Laboratory Studies
- Blood tests at Karolinska University Hospital in a jointly run laboratory
- Performance studies in simulated clinical scenarios using a hybrid mock circulation simulator (simulating the human body) at KU Leuven under the direction of Dr. Libera Fresiello
- Blood flow pattern analysis using 4D MRI in collaboration with Linköping University
- Endurance tests of components and the whole TAH system in custom made test rigs at Realheart’s laboratory in Västerås
Anatomical and Animal Studies
- Anatomy studies in humans under the direction of Professor Bart Meyns at KU Leuven
- Short-term and long-term studies in sheep at Medanex Clinic in Belgium
Clinical Trials
As artificial hearts are not a common product category the description below is the current best estimate of the route to market. However, based on Realheart’s communication with the notified body and regulatory authorities the demands may be updated. There is a great clinical need, and it is in everyone’s interest to bring innovative products that can improve quality of life to the market as swiftly as possible.
Small Clinical Trial in Patients
This trial will form the first evaluation of the product for the treatment of heart failure in human patients. The number of patients will be decided by the relevant authorities. Realheart is already planning for these trials by identifying collaborating physicians and hospitals.
Large Clinical Trial in Patients
Following the small clinical trial, Realheart will work with several physicians and hospitals in parallel to speed up the patient recruitment process. The number of participating patients will be determined by the authorities.
Product Launch
After the authorities evaluate data from the large clinical trial and assess the safety of the device, Realheart will seek regulatory permission to launch the product on the market.

Collaborations
In addition to collaboration with institutions and clinics, Realheart works with an international network of expert heart surgeons, veterinarians, engineers, and biomedical researchers. In 2020, a scientific advisory board was established to further support Realheart’s medical and technical competencies. You can read more about the medical advisory board here.
Scientific Documentation
All preclinical studies are carefully documented. This documentation is necessary to obtain approval to begin clinical trials in humans. Additionally, these results are often published in scientific articles in leading journals. You can read more about Realheart’s journal articles here.