Press Release 18 November, 2022
Summery of Interim Report
Group Overview
Third Quarter 2022
(2022-07-01 – 2022-09-30)
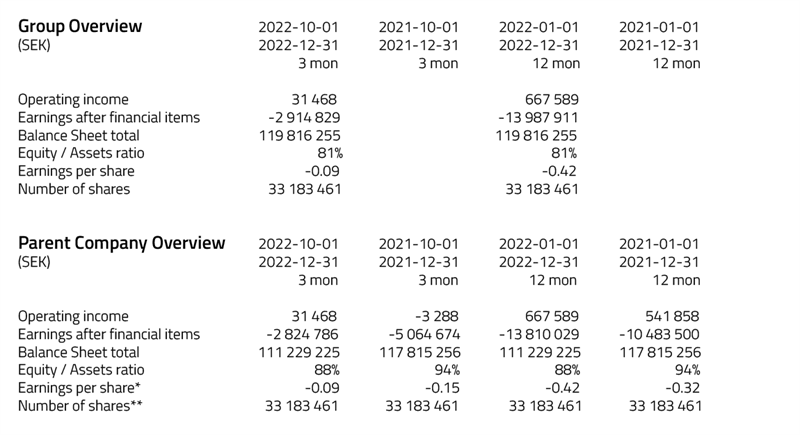
• Operating income amounted to 14 365 624 SEK.
• Earnings after financial items amounted to
2 998 939 SEK.
• Balance sheet total,122 003 532 SEK.
• Earnings per share * amounted to SEK 0.09.
• Equity / assets ratio was 95%.
Parent Company Overview
First to Third Quarter 2022
(2022-01-01 – 2022-09-30)
• Operating income amounted to 14 375 624 (545 146) SEK.
• Earnings after financial items amounted to 3 086 779 (- 5 418 826) SEK.
• Balance sheet total, 121 112 466 (140 666 993) SEK.
• Earnings per share * amounted to SEK 0.09 (-0.16) SEK.
• Equity / assets ratio was 94% (40%).
Third Quarter 2022
(2022-07-01 – 2022-09-30)
• Operating income amounted to 13 471 835 (16 445) SEK.
• Earnings after financial items amounted to 8 997 861 (-1 410 135) SEK.
• Balance sheet total, 121 112 466 (140 666 993) SEK.
• Earnings per share * amounted to SEK 0.27 (-0.04) SEK.
• Equity / assets ratio was 94% (40%).
* Earnings per share: Earnings for the period divided by 33 183 461.
** Equity ratio: Equity divided by total capital.
Amounts in parentheses refer to the corresponding period last year.
Significant Events During the Third Quarter of the Year
• July begins with a successful second implantation of the clinical version of Realheart TAH in sheep. The important milestone of 24-hour survival is achieved.
• At the end of August, one of Realheart's biomedical engineers presents the company's work on developing methods to measure blood damage in pulsatile pumps. This at a conference on blood damage caused by heart pumps, organised by the University of Rostock in Germany.
• September begins with the Realheart team attending the European Society of Artificial Organs (ESAO) conference in Austria. Three of the company's engineers present their research while the CEO and CTO are on hand to make contacts and represent the company.
• In the last days of September, the Company announces that the third implantation on animals is scheduled to take place in October.
Significant Events After the End of the Period
• Three days into October, Realheart's CEO appears at FOKUS Patient, organised by Fokus Patient in collaboration with the Gift of Life and the European Society of Organ Transplantation, ESOT. The presentation is entitled ''When organs are not enough'' in the block on the future of organs and organ donation. Another speaker in the same session was Dr Bartley Griffith, an American surgeon who recently performed a high profile pig heart transplant in a human heart failure patient.
• Midway through October, Realheart is granted an additional loan from Almi of SEK 7.6 million, which will ensure continued rapid product development targeting clinical trials.
Revenue and Result
Scandinavian Real Heart is currently working with research and development and currently has no sales of any products. The turnover reported for the period consists of grants received. Research and development costs of Realheart® TAH were capitalized during Q3 with 15.9 million. During the period, write-downs of capitalized costs for research and development were made by 0 million.
The item other external costs of 19.9 million consists of costs for purchased services of 8.9 million and various other costs of SEK 11.0 million. Of these costs, 15.9 million has been capitalized.
Financial Position
Following the rights issue completed in 2021, the Company is now in a strong liquidity position with approx. SEK 22 million. Together with the grant of SEK 25.7 million that we have been granted from the EIC and which we will receive in 2022/2024, we have funding that will last into quarter 2, 2023.
In order to solve the Company's longer-term financing needs, Realheart works continuously to evaluate alternatives for further capitalization of the Company.
CEO Ina Laura Perkins has the Word
The development of Realheart® TAH continued with a high level of activity throughout the summer. We continued to work on our virtual implantations where we have now increased the number of patients to 15, and the pump has proven to be compatible with all male patients' bodies. The virtual implantations have also enabled us to start defining the dimensions for our follow-up product MiniHeart, which is intended for female patients among others. Nice synergies, in other words.
In July, we performed the second implantation on sheep and reached the milestone of 24 hours survival, which was extremely satisfying for the whole team. The pump did what it was supposed to do and the implantation proceeded smoothly, again providing a beneficial short time on the heart-lung machine before the complicated aftercare of the animal took place. Now that we are past the first critical day, we aim to gradually increase the survival time of each operation – and in parallel fine-tune the software of the controller based on the data we collect.
Something else I carry with me from this quarter is our participation in the ESAO conference in Austria. It brought together many of those who could be our future key customers and partners, and we had many fruitful discussions with representatives of the international clinical community, where we were able to learn about their experiences in treating patients with the artificial hearts available today. Several cardiac surgeons expressed an interest in our work, and several offered to help in various ways, such as with clinical trials.
“Implanting a TAH is the easiest thing you can do, you press a button and the patient is stable”, said one doctor, and we found that the main obstacle to the use of artificial hearts is the risk of side effects and the need for continued care. There is no product that combines easy implantation with reduced blood damage and that gives the patient a high quality of life – but that is only a few years away now.
As we know, we have identified blood damage reduction as one of our key success factors, so it is particularly interesting to see how our competitors are working. During the conference, we were able to hear a presentation from a company that has chosen to have an external consultant perform their tests on blood from cows. We carry out our tests on human blood in our own laboratory – something few others have access to – and because human blood is three times more sensitive than that of cows, we expect to get more reliable answers on how the heart pump will perform in the human body.
In return, some of our engineers had the opportunity to present their work related to our testing activities to the conference participants. We have, as I often point out, a team with a unique collective expertise and I was really proud to see the great interest in Soteris Andreous, Joseph Bornoff and Faisal Zaman's methodology for durability, simulation and blood testing. We also received an award for our collaboration with the University of Bath.
The interest from students who want to collaborate with us is very high, and I see this as a channel for continued future recruitment of high-skilled talent. We currently have an MSc student from the University of Twente in the Netherlands who is working on making our blood testing rig more user-friendly. This will help tremendously as we perform tests every week and will continue to do so throughout the development process. Furthermore, our students from Uppsala University and the Royal Institute of Technology continue their work with us.
In August, our new CFO Jonas Caspari Bark joined us. Jonas will have a very important role as a unifying force at our headquarters in Västerås, as I am in the blood lab in Stockholm or abroad a lot of the time. We are building a good structure for the company so that I can focus on the right things as we continue our journey towards clinical trials and the market.
Ina Laura Perkins
CEO, Scandinavian Real Heart AB
Scandinavian Real Heart AB
Swedish innovation power has given the world medical technology inventions such as the heart and lung machine, the pacemaker and the dialysis machine. The next big innovation is Realheart's artificial heart. A Swedish patented innovation that will save the lives of heart failure patients. Every year, almost 3,500 people die of heart failure in Sweden alone. Today, the only rescue is a heart transplant, but the number of donated hearts is only enough for 2% of those in need.
The idea behind Realheart was born in 1999, when the doctor and innovator Azad Najar made the first sketch of an artificial heart that fully mimics the biological one. In 2007, Azad co-founded Scandinavian Real Heart with two partners. The original idea behind Realheart® TAH is based on flow analyzes made at KTH 2002- 2005 and is based on constructing an artificial heart that mimics the biological. By imitating its basic principle, a pressure and flow is created with the aim to reduce the risk of blood clots and provides an energy-efficient blood flow. These factors are important to give the patient a good quality of life. The development of the product has progressed strongly over the years. Blood circulation, blood pressure, oxygen saturation, pump function and pulse generation have been verified in ethically approved animal experiments. Today, research and development takes place in close collaboration with world-leading heart surgeons, researchers and engineers.
Patent Protection
Realheart has granted patents in Sweden, Germany, the United Kingdom, the United States, China and India that protect the original pump principle in TAH. This patent also provides protection for future products: RealVAD® and PulsePump®.
Patent protection is also available on the latest version of Realheart® TAH in Sweden, USA, UK, Australia and Japan. The patent application for it has also been filed for Germany and Canada. The patents provide protection in the markets that are largest and most important for artificial hearts right now, with the exception of China and India which are considered important emerging markets. In addition to the patent protection described above, Realheart has also approved patents in Sweden, the USA and the United Kingdom for the future Sternal prosthesis product. The application is also submitted in Germany and France. In 2018, a new connection was designed for a simple and secure connection between Realheart® TAH and the body's circulatory system. The patent application for this has also been filed.
Finally, the patent application has been filed in two parts for the use of pressure sensors for the automatic control. Given the existing patents together with the new patent applications, the Board believes that the company has a strong patent situation and strong intellectual property protection.
Mission and Goal
Realheart's mission is to use medical technology solutions to save as many heart failure patients as possible and to create the best conditions for a life-affirming continuation of life. The company's overall goal is for the artificial heart to be commercialized and become a full-fledged treatment alternative for patients with heart failure. The heart should have a better function than the solutions that are on the market today. It should be possible to use both as a bridge to transplantation and as final therapy.
The Stock
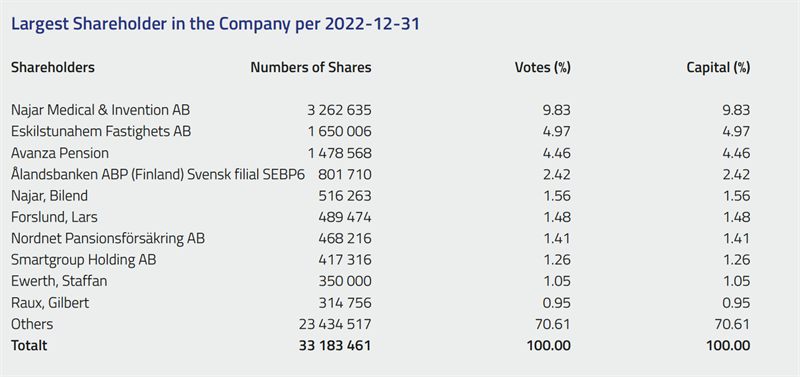
Scandinavian Real Heart AB was listed on the Nasdaq First North Growth Market in December 2021. Nasdaq First North GM is a registered SME marketplace for growth companies that enables Nordic and international entrepreneurs to gain access to growth capital to develop and expand their operations. As of September 30, 2022, the number of shares in Scandinavian Real Heart was 33,183,461.

Principles for the Preparation of the Interim Report
The interim report has been prepared in accordance with the Annual Accounts Act and with the application of general advice, recommendations and statements from the Swedish Accounting Standards Board.
Audit Review
The interim report has not been reviewed by the Company's auditor.
Upcoming Financial Reports
Year-end Report, 2022 2023-02-24
Interim Report Q1, 2023 2023-05-18
Submission of Interim Report
Västerås, November 18, 2022
The Board
Scandinavian Real Heart AB
For Further Information, Please Contact
Ina Laura Perkins
CEO Scandinavian Real Heart
Phone: +46 70 406 49 21
E-mail: inalaura.perkins@realheart.se
Jonas Caspari Bark
CFO Scandinavian Real Heart
Mobile +46 70 643 88 61
E-mail: jonas.bark@realheart.se
This disclosure contains information that Real Heart is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information in this press release has been published through the agency of the contact persons set out below, at the time stated by Scandinavian Real Heart AB’s news distributor Cision upon publication of this press release.
Link to archive for financial reports: https://realheart.se/sv/investerare/arkiv/