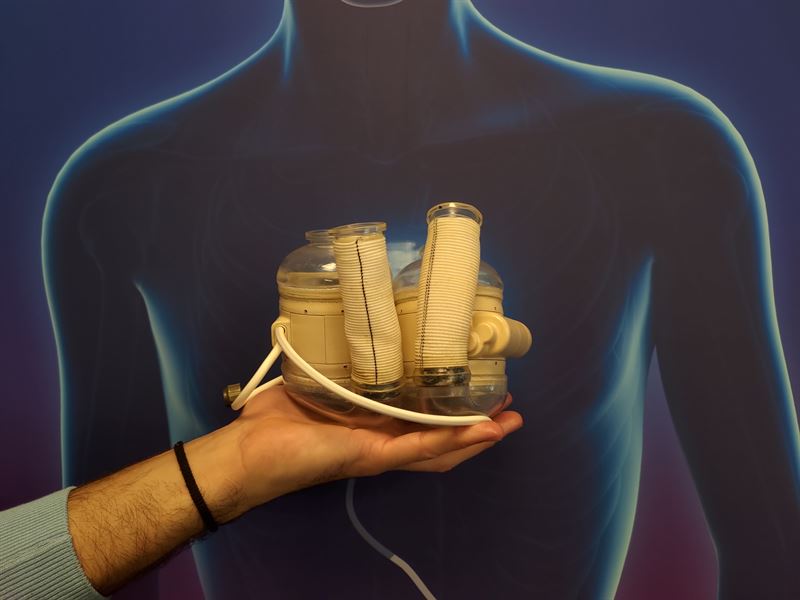
Realheart has initiated collaborations with Lisa Prahl Wittberg and Seraina Dual, both researchers with expertise in heart pumps at the Royal Institute of Technology, KTH, in Stockholm. These collaborations bring additional expertise to the company in the areas of blood testing and evaluation of the control unit for the clinical version of the Realheart TAH, as well as in the company's research on new products.
Realheart collaborates with a number of international scientists at various institutes and universities developing the world's first four-chamber artificial heart. Through these new collaborations, the company aims to strengthen its Swedish network to benefit from it both in the development of Realheart TAH and other future products.
''At present we recruit mostly internationally to find the skills we need for our growth, but it would be beneficial to be able to find them at home in the future. We want to strengthen Sweden's international competitiveness in research and development of heart pumps. It is therefore an advantage if students learn about artificial hearts and the specialist skills required in this field early on in their education," said Realheart CEO Ina Laura Perkins.
The core of Professor Lisa Prahl Wittberg's research is flow analysis with a focus on complex fluids in the human body, such as blood. In particular, she focuses on blood flow and the risk of blood clots occurring in the body or in pumps, cannulas and other components used in the treatment of critically ill patients The research is conducted in close collaboration with clinical partners.
Seraina Dual is Assistant Professor in the Department of Biomedical Engineering. Her research focuses on the use of sensor systems to predict, prevent, and cure cardiovascular diseases. Her work has shown how heart pumps should be used in patients with smaller bodies, such as women, and how a combination of different sensors could help.
Realheart has collaborated with KTH before. When founder Azad Najar began sketching what would become Realheart in 1999, the basic idea was to develop an artificial heart that mimics the human heart. This idea was based on flow analyses conducted by Professor Said Zahrai at KTH.
''We've come a long way since then and it is great to now return to KTH for continued collaboration as we move towards clinical trials. Seraina's vast expertise will be invaluable in further improving the equipment we use for the blood tests, and with Seraina's help we will be able to subject the controller to even more advanced stress tests to ensure it is safe even under the most stressful conditions in the human body,'' said Ina Laura Perkins.