Revenue and Result
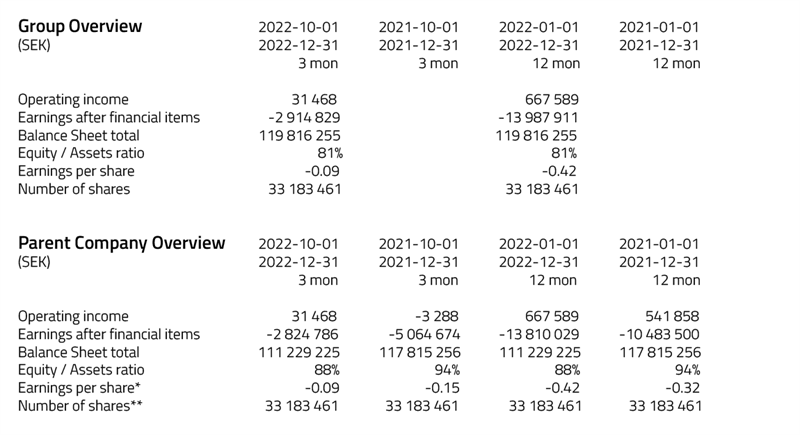
Scandinavian Real Heart is working with research and development and currently has no sales of any products. The income reported for the period consists mainly of foreign currenty exhance gains. Research and development costs of Realheart® TAH were capitalized during the year with 4.1 MSEK. During the period, write-downs of capitalized costs for research and development were made by 0 MSEK.
The item other external costs of 4.2 MSEK consists of costs for purchased services of 1.0 MSEK and various other costs of 3.2 MSEK. Of these costs, 4.1 MSEK has been capitalized.
Financial Position
During the period, the company has received cash of 3.7 MSEK from an extended loan from ALMI and 4.1 MSEK from TO1. With a cash balance of 10.5 MSEK, the companty has funding that will last into third quarter of 2023.
In order to solve the Company's longer-term financing needs, Realheart works continuously to evaluate alternatives for further capitalization of the Company.
Significant Events During the First Quarter of the Year
January starts with communicating increased survival time from one to four days, and also that several performance criteria were increased. All this was reached in the end of 2022, but was communicated in 2023, after collection of data including analysis. The main citieria were: no hemolysis, no thromboembolic events, high pumping capacity and good right-left balance, and short procedure.
In January, the company also communicates that they, together with KTH, developed Sweden's First Patient Simulator. The collaboration between Realheart and KTH to develop the simulator (scientific term: "hybrid simulator") began in 2022 after a contribution of SEK 4 million from Smart Elektronik, a joint initiative by Vinnova, Formas and the Swedish Energy Agency.
In Januari, the company also communicates that Dr. Ulf Kjellman Strengthens Realheart's Medical Advisory Board. Ulf Kjellman has over 35 years of experience in cardiothoracic surgery.
End of February, Professor Bart Meyns Strengthens Realheart's Advisory Board. Dr Meyns is a Professor at the University of Leuven in Belgium and is also leading Realheart's surgical team in the ongoing animal study.
In the beginning of March, Realheart announces the outcome of the exercise of warrants of series TO1. A total of 7 183 148 warrants of series TO1 were exercised, corresponding to approximately 70 percent of the total number of warrants, which brings the Company approximately SEK 4.2 million before issuing related costs.
Last days of March, cardiac surgeon Ulf Kjellman Takes over as Chief Medical Officer (CMO) at Realheart. Kjellman, who has many years of experience in advanced cardiac surgery, will be clinically responsible for the company's preclinical and clinical studies.
The first quarter ends with Realheart's CEO to Present at the Swiss Nordic Bio Zürich Healthcare Investor Conference. There is an increased interest in the possibilities of total artificial hearts (TAH) and the interest in Realheart is much higher this year compared to last year's conference. Artificial hearts open the door to a paradigm shift in the care of patients with severe heart failure. Here, saving lives and reducing healthcare costs go hand in hand.
Significant Events After the end of the Period
In the middle of April the company communicates that the market need in the US and Europe is estimated at 200,000 artificial hearts per year – equivalent to SEK 200 Billion – and the trend is accelerating. Industry players and Realheart estimate that the market potential for total artificial hearts (TAHs) is over 200,000 units per year in Europe and the US. Realheart believes it is well positioned to take significant market share with its four-chamber artificial heart.
In April, Joseph Bornoff’s Simulations of the Realheart is Published in the Springer Nature Journal Scientific Reports. It is an advanced computational fluid dynamics (CFD) model of the Realheart® TAH (Total Artificial Heart) to study blood flow in the pump and optimize its function. The work has been co-authored with colleagues at Realheart.
At the end of April, results of Scandinavian Realheart’s Total Artificial Heart demonstrating lower rates of hemolysis are published. The peer-reviewed study titled ”In Vitro Hemolytic Performance of the Realheart V11C TAH Prototype with Porcine Blood” was published in the journal of Artificial Organs.
During the first days in May, scientists at Scandinavian Real Heart and at the University of Bath publish the world’s first computer simulation method for dual mechanical heart valves in series. The article “Overset meshing in combination with novel blended weak-strong fluid-structure interactions for simulations of a translating valve in series with a second valve” was published in the scientific journal “Computer
CEO Ina Laura Perkins has the Word
I am pleased to look back at a busy and very successful first quarter of 2023 with several key achievements for Realheart.
First, and foremost, by reaching the four-day survival benchmark of animals implanted with our TAH, we have shown that the Company is on track towards obtaining approval for the first in human use of our implant. This is the result of significant efforts by our R&D team, surgeons, and others who continue to improve surgical methodology and study protocols to a level where the procedure is becoming routine.
Similarly, our R&D team together with researchers at KTH Royal Institute of Technology, successfully developed the first patient simulator (digital twin) that is used to test the Company’s TAH algorithm. Apart from this being a “first” in our space, having access to digital twin technology will improve and streamline our R&D processes for current and future products. The project was rewarded with a grant from the Strategic Innovation Program Smarter Electronic Systems – a joint initiative by Vinnova, Formas and the Swedish Energy Agency.
We have continued to expand our team with two world renowned cardiac surgeons: Dr. Ulf Kjellman has been appointed as the Company’s CMO, and Prof. Bart Meyns has joined our Medical Advisory Board. Both are Key Opinion leaders with extensive experience in cardiac surgery and from the field of developing and testing heart pumps.
With the support of our shareholders, we have successfully raised 4.2 MSEK from warrants of series TO1. In an increasingly competitive and rapidly evolving environment, this as well as future financing, are critical for us to continue our current trajectory of successes, to effectively progress the development of the Realheart® TAH into the clinic towards product approval, and to create shareholder value.
We have continued to promote Realheart on the international stage to both investors and the medical and scientific community. Dr Azad Najar, our founder and Chief Innovation Officer, was selected as keynote speaker at the Artificial
Intelligence (AI) Conference in Cairo and the Dubai Innovation Conference.
And I have, together with Dr Oliver Voigt, presented Realhart at the Healthcare Investor Conference, Swiss Nordic Bio, in Zürich. Creating awareness and an international footprint of our technology among investors and the medical and scientific communities is key to the future success of the Company.
Significant events after the end of the period is that Realheart has published three scientific articles, in Springer Nature journal Scientific Reports, the journal Artificial Organs, and Computer Methods in Biomechanics and Biomedical Engineering . This is really important work, done in international collaboration between scientists at Realheart and researchers at the University of Bath and Swansea University Medical School, both in the United Kingdom.
After an eventful start of the year, we are incredibly excited and full of confidence. Knowing that we are on the right track, we are looking forward to completing our pre-clinical studies. Again, I want to highlight that support from current and future shareholders is essential for us to continue on our path to success.
Ina Laura Perkins
CEO, Scandinavian Real Heart AB
Scandinavian Real Heart AB
Swedish innovation power has given the world medical technology inventions such as the heart and lung machine, the pacemaker and the dialysis machine. The next big innovation is Realheart's artificial heart. A Swedish patented innovation that will save the lives of heart failure patients. Every year, 3,500 people die of heart failure in Sweden alone. Today, the only rescue is a heart transplant, but the number of donated hearts is only enough for 2% of those in need.
The start-up of the company was initiated by the doctor Azad Najar in 1999 when he started sketching an artificial heart that completely mimics the biological. In 2007, Azad co-founded Scandinavian Real Heart with two partners. The original idea behind Realheart® TAH is based on flow analyzes made at KTH 2002-2005 and is based on constructing an artificial heart that mimics the biological. By imitating its basic principle, a pressure and flow is created that reduces the risk of blood clots and provides an energy-efficient blood flow. These factors are important to give the patient a good quality of life. The development of the product has progressed strongly over the years. Blood circulation, pump function, pressure, and pulse generation have been verified in ethically approved animal experiments. Today, research and development takes place in close collaboration with world-leading heart surgeons, researchers and engineers.
Patent Protection
Realheart has granted patents in Sweden, Germany, the United Kingdom, the United States, China and India that protect the original pump principle in TAH. This patent also provides protection for future products: RealVAD® and PulsePump®.
Patent protection is also available on the latest version of Realheart® TAH in Sweden, USA, UK, Australia and Japan. The patent application for it has also been filed for Germany and Canada. The patents provide protection in the markets that are largest and most important for artificial hearts right now, with the exception of China and India which are considered important emerging markets. In addition to the patent protection described above, Realheart has also approved patents in Sweden, the USA and the United Kingdom for the future Sternal prosthesis product. The application is also submitted in Germany and France. In 2018, a new connection was designed for a simple and secure connection between Realheart® TAH and the body's circulatory system. The patent application for this has also been filed.
Finally, the patent application has been filed in two parts for the use of pressure sensors for the automatic control. Given the existing patents together with the new patent applications, the Board believes that the company has a strong patent situation and strong intellectual property protection.
Mission and Goal
Realheart's mission is to use medical technology solutions to save as many heart failure patients as possible and to create the best conditions for a life-affirming continuation of life. The company's overall goal is for the artificial heart to be commercialized and become a full-fledged treatment alternative for patients with heart failure. The heart should have a better function than the solutions that are on the market today. It should be possible to use both as a bridge to transplantation and as final therapy.
The Stock
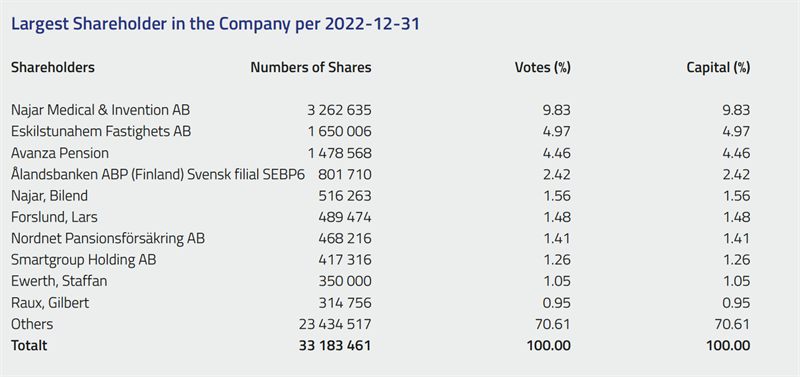
Scandinavian Real Heart AB was listed on the Nasdaq First North Growth Market in December 2021. Nasdaq First North GM is a registered SME marketplace for growth companies that enables Nordic and international entrepreneurs to gain access to growth capital to develop and expand their operations. As of March 31, 2023, the number of shares in Scandinavian Real Heart was 34 979 248.

Principles for the preparation of the Interim Report
The interim report has been prepared in accordance with the Annual Accounts Act and with the application of general advice, recommendations and statements from the Swedish Accounting Standards Board.
Audit Review
The interim report has not been reviewed by the Company's auditor.
Upcoming Financial Reports
Annual Report 2022 2023-05-26
Interim Report Q2, 2023 2022-08-24
Interim Report Q3, 2023 2022-11-16
Year-end Report, 2023 2023-02-15
Annual General Meeting
The company's annual general meeting is planned to be held Friday, June 16th.
Submission of Interim Report
Västerås, May11, 2023
The Board
Scandinavian Real Heart AB
For Further Information, Please Contact
Ina Laura Perkins
CEO Scandinavian Real Heart
Phone: +46 70 406 49 21
E-mail: inalaura.perkins@realheart.se
Jonas Caspari Bark
CFO Scandinavian Real Heart
Phone: +46 70 643 88 61 E-mail: jonas.bark@realheart.se
This disclosure contains information that Real Heart is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information in this press release has been published through the agency of the contact persons set out below, at the time stated by Scandinavian Real Heart AB’s news distributor Cision upon publication of this press release.
Link to archive for financial reports: https://realheart.se/sv/investerare/arkiv/