Press Release 24 February, 2023
Summary of the Year-end Report
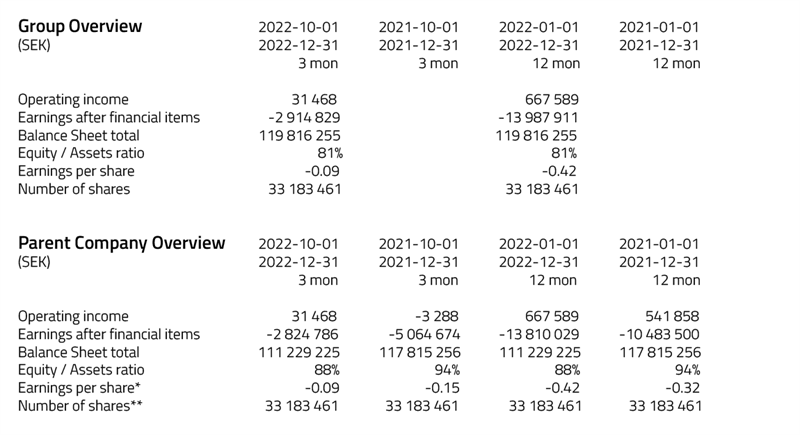
* Equity ratio: equity divided by total capital.
** Earnings per share: profit for the period divided by 33 183 461.
Proposed Treatment of the Company's Retained Earnings
The Board of Directors propose that the company's earnings be carried forward in the amount of 5 873 671.

No dividends will be paid to shareholders.
Revenue and Results
Scandinavian Real Heart is working with research and development and currently has no sales of any products. The income reported for the period consists mainly of foreign currenty exhance gains. Research and development costs of Realheart® TAH were capitalized during the year with 21.2 MSEK. During the period, write-downs of capitalized costs for research and development were made by 0 MSEK.
The item other external costs of 24.5 MSEK consists of costs for purchased services of 10.2 MSEK and various other costs of 14.3 MSEK. Of these costs, 21.2 MSEK has been capitalized.
Financial Position
With a cash balance of 11.3 MSEK, the companty has funding that will last into second quarter of 2023. In order to solve the Company's longer-term financing needs, Realheart works continuously to evaluate alternatives for further capitalization of the Company.
Significant Events During the Fourth Quarter of the Year
• In early October, Realheart's CEO Ina Laura Perkins will appear at the Transplantation Forum organised by Focus Patient in collaboration with The Gift of Life and the European Society of Organ Transplanta- tion, ESOT, at the City Conference Center in Stock- holm. Among the speakers will be Dr Bartley Griffith, an American surgeon who recently performed a high-profile pig heart transplant for a human.
• At the end of October, it is announced that Realheart's artificial heart is featured in a German TV documentary on heart disease and new scientific developments, broadcast on ZDF on 25 October. Both Realheart's founder Azad Najar and Dr Dilek Gürsoy, who is part of the company's surgical team for the ongoing animal studies, are interviewed in the programme.
• The last thing to happen in October is that the third implantation on animals is completed and trials continue. Once again, the implantation went quickly and progress was made, among other things, in the optimal placement of the pump.
Significant Events After the End of the Period
• A step into January, the company reaches a new milestone with Realheart TAH. Since the series of animal trials with the clinical version of the company's artificial heart began in 2022, Realheart has been able to progressively increase survival time from the previous milestone of one day to four days. After implantation, the animal has been able to stand up and eat. In addition, several key performance criteria were confirmed.
• In the second half of January, a press release announces that Realheart and KTH are optimising heart function in Sweden's first patient simulator. The collaboration between Realheart and KTH to develop the simulator (scientific term: "hybrid simulator") started in 2022 after a grant of SEK 4 million from Vinnova Smart Elektronik.
• At the end of January, it is announced that Dr. Ulf Kjellman strengthens Realheart's medical council. Ulf Kjellman has over 35 years of experience in cardiac and thoracic surgery and was involved in the introduction of HeartMate II, HeartMate 3, Centrimag, Maquet pumps, Berlin Excor and SynCardia TAH, among others.
• The last event of January is that the company is offering subscription right holders the right to subscribe to HEART TO1. The subscription period will run from 1-28 February 2023.
CEO Ina Laura Perkins "Our heart surgeons speak warmly about our artificial heart"
In the last three months of the year, we made progress in three areas: international marketing, soft funding and our animal studies. In October, our Realheart® TAH was featured in prime time on the German TV channel ZDF in a documentary on heart disease.
A German TV crew visited one of our surgeries to interview the company's founder Azad Najar and the heart surgeon Dilek Gürsoy who performs the operations. Interviewer was a German celebrity chef who has a heart condition himself, and the show followed a young woman with heart failure on her road to a successful heart transplant. The documentary highlights Realheart as a promising alternative solution to the organ shortage.
Germany is one of the largest markets in Europe for artificial hearts. To learn more, I visited the clinic that performs the most heart pump surgeries in the country: the Medical University of Hannover. There I interviewed heart surgeon Professor Jan Schmitto, who said that in Germany alone, 40,000-60,000 artificial hearts are needed each year. This means that we have underestimated the need.
Proof of the strong need is the sales forecast for 2023 from our European competitor. They started selling in November and expect to sell over 100 hearts in the first year at revenues of €10-13 million and scale up production to 1000 hearts by 2027. This is great news for heart failure patients in Europe, but it is only enough for a fraction of patients. Doctors are reminding us that even more artificial hearts are urgently needed.
The situation is similar in the rest of the world. In December, I spoke at the Critical Heart Disease Congress in China. The digital conference has so far been viewed by 24,000 viewers, including doctors and healthcare professionals from Australia, the UK, Austria, Italy, the US and China. I also gave an introduction to the International Society for Mechanical Circulatory Support (ISMCS), for which I have been on the Board since May. Through these networks, we can find the right partners and future customers faster, and raise awareness of artificial hearts as a cure for heart failure. The marketing has already had an impact as I have received enquiries from doctors in Brazil and elsewhere.
To start clinical trials, we need good results from animal studies in addition to blood and durability tests. During the year, we have worked on the surgical process and animal care, for example on the settings of the heart-lung machine. We have realized that this is not readily available knowledge but that we are building up valuable unique know-how within the team. Therefore, we decided not to communicate as frequently and in as much detail about our animal studies as we had done in the past. I understand that this may frustrate shareholders, but I can assure you that we have quietly made great progress: 4 days of survival without blood damage.
Developing an artificial heart requires excellence throughout the entire chain – from idea to marketing and sales. This chain is complete with us, we have all the pieces needed to get to market. Here, our international expertise from several artificial heart projects contributes greatly, as it is a challenge to find equivalent in Sweden. I am convinced that our diversity contributes strongly to our success. It is awesome that we have over 100 years of combined expertise.
In addition to a strong team and good results, continued funding is needed to reach the market. Therefore, we are working on the due diligence process for the European Innovation Council fund which has nominated us for €15 million investment, as well as on finding soft funding. We are very grateful that ALMI chose to continue supporting our work with an innovation loan of 7.6 million SEK. In the first quarter of 2023, our shareholders can further strengthen our cash position via the share option. The funding will be used for animal studies and to start the blood tests on the clinical version of Realheart TAH – we are proceeding nicely according to our plan in other words.
Scandinavian Real Heart AB
Swedish innovation power has given the world medical technology inventions such as the heart and lung machine, the pacemaker and the dialysis machine. The next big innovation is Realheart's artificial heart. A Swedish patented innovation that will save the lives of heart failure patients. Every year, almost 3,500 people die of heart failure in Sweden alone. Today, the best treatment is a heart transplant, but the number of donated hearts is only enough for 2% of those in need.
The idea behind Realheart was born in 1999, when the doctor and innovator Azad Najar made the first sketch of an artificial heart that fully mimics the biological one. In 2007, Azad co-founded Scandinavian Real Heart with two partners. The original idea behind Realheart® TAH is based on flow analyzes made at KTH 2002-2005 and is based on constructing an artificial heart that mimics the biological. By imitating its basic principle, a pressure and flow is created with the aim to reduce the risk of blood clots and provides an energy-efficient blood flow. These factors are important to give the patient a good quality of life. The development of the product has progressed strongly over the years. Blood circulation, blood pressure, oxygen saturation, pump function and pulse generation have been verified in ethically approved animal experiments. Today, research and development takes place in close collaboration with world-leading heart surgeons, researchers and engineers.
Patent Protection
Realheart has granted patents in Sweden, Germany, the United Kingdom, the United States, China and India that protect the original pump principle in TAH. This patent also provides protection for future products: RealVAD® and PulsePump®.
Patent protection is also available on the latest version of Realheart® TAH in Sweden, USA, UK, Australia and Japan. The patent application for it has also been filed for Germany and Canada. The patents provide protection in the markets that are largest and most important for artificial hearts right now, with the exception of China and India which are considered important emerging markets. In addition to the patent protection described above, Realheart has also approved patents in Sweden, the USA and the United Kingdom for the future Sternal prosthesis product. The application is also submitted in Germany and France. In 2018, a new connection was designed for a simple and secure connection between Realheart® TAH and the body's circulatory system. The patent application for this has also been filed.
Finally, the patent application has been filed in two parts for the use of pressure sensors for the automatic control. Given the existing patents together with the new patent applications, the Board believes that the company has a strong patent situation and strong intellectual property protection.
Mission and Goal
Realheart's mission is to use medical technology solutions to save as many heart failure patients as possible and to create the best conditions for a good quality of life. The company's overall goal is for the artificial heart to be commercialized and become a full-fledged treatment alternative for patients with heart failure. The heart should have a better function than the solutions that are on the market today. It should be possible to use both as a bridge to transplantation and as permanent therapy.
The Stock
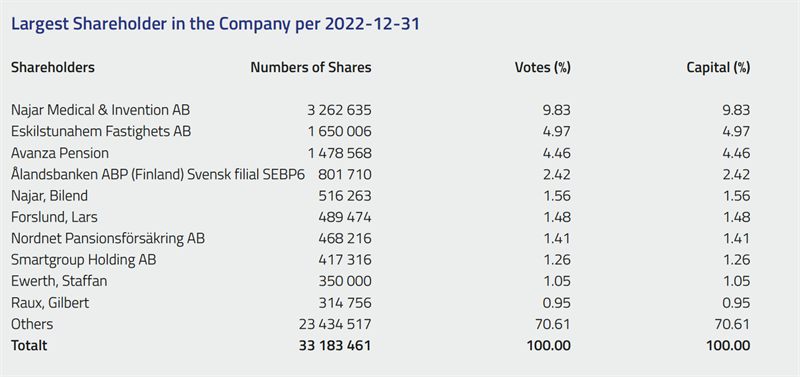
Scandinavian Real Heart AB was listed on the Nasdaq First North Growth Market in December 2021. Nasdaq First North GM is a registered SME marketplace for growth companies that enables Nordic and international entrepreneurs to gain access to growth capital to develop and expand their operations. As of September 30, 2022, the number of shares in Scandinavian Real Heart was 33,183,461.
Principles for the Preparation of the Interim Report
The interim report has been prepared in accordance with the Annual Accounts Act and with the application of general advice, recommendations and statements from the Swedish Accounting Standards Board.
Audit Review
The interim report has not been reviewed by the Company's auditor.
Upcoming Financial Reports

Submission of Interim Report
Västerås, Februari 24, 2023
The Board
Scandinavian Real Heart AB
For Further Information, Please Contact
Ina Laura Perkins
CEO Scandinavian Real Heart AB
Phone: +46 70 406 49 21
E-mail: inalaura.perkins@realheart.se
Jonas Caspari Bark
CFO Scandinavian Real Heart AB
Mobile +46 70 643 88 61
E-mail: jonas.bark@realheart.se
This disclosure contains information that Real Heart is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information in this press release has been published through the agency of the contact persons set out below, at the time stated by Scandinavian Real Heart AB’s news distributor Cision upon publication of this press release.
Link to archive for financial reports: https://realheart.se/sv/investerare/arkiv/















